Why ImmunoSpot®?
Reliable Detection of Rare Antigen-Specific Lymphocytes in Blood
In general, ELISPOT and FluoroSpot assays (collectively called ImmunoSpot® assays) are suited to visualize, at single-cell resolution, the secretion of any type of molecule (“analyte”) by any type of cell. In particular, ImmunoSpot® assays are primarily used for ex vivo immune monitoring purposes due to their unique ability to detect even very rare antigen-specific lymphocytes in fresh or cryopreserved peripheral blood mononuclear cells (PBMC), biopsies, or other primary cell material. High reproducibility of results, very efficient cell utilization, relatively little labor involved, high-throughput capability, comparatively low cost for kits and instrumentation, automated objective and auditable data analysis, and the ease to validate such assays for regulated testing, all contribute to the success of the ImmunoSpot® platform (see Ref).
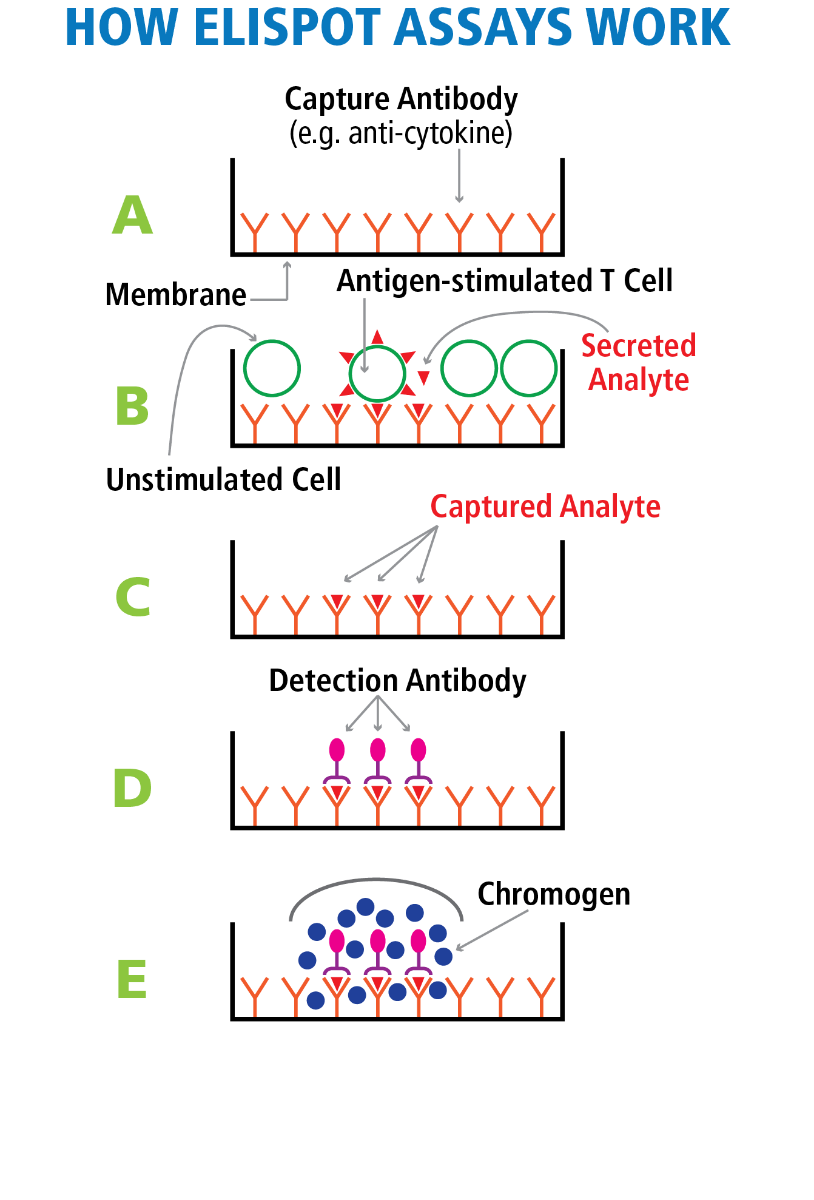
T Cell ImmunoSpot® Assays detect cytokines (and/or cytolytic effector molecules such as granzyme B or perforin) that antigen-specific CD4+ or CD8+ T lymphocytes release upon antigen encounter. The T Cell ImmunoSpot® test relies on a PVDF membrane that has been pre-coated with an analyte (e.g., interferon-gamma, IFN-γ) specific “capture antibody” (Panel A in the Figure). Coating with several capture antibodies permits the simultaneous detection of several analytes in multi-color ImmunoSpot® formats.
In the first step of T Cell ImmunoSpot® assays, a monolayer of PBMC (or other primary cell material possibly containing T cells specific for the test antigen) is plated in 96-well (or 384-well) format onto a sensitized membrane. Addition of the nominal test antigen/peptide results in the activation of the antigen-specific T cells in the corresponding well, if such T cells are present, triggering them to produce analyte (e.g. IFN-γ). As the analyte is secreted, it is immediately captured on the membrane around each secreting cell (Panel B of the Figure). After this cell culture phase of the assay is terminated, the cells are removed (they survive the assay unharmed and can be re-tested) leaving the secretory footprint of each antigen-specific T cell behind on the membrane (Panel C of the Figure). Next, the membrane-bound analyte spots (“spot forming units”, SFU) are visualized using analyte-specific “detection antibodies” (Panel D). This is accomplished by either enzymatic reactions generating precipitating substrates that are visible with white light (single- or double-color ELISPOT, Panel E) or by using multiple fluorescent tags (multi-color FluoroSpot). By counting the SFU per well, the number/frequency of antigen-specific T cells within the tested PBMC can be determined (see Ref). Measuring several T cell analytes in multicolor FluoroSpot format permits to define the effector lineage and differentiation state of the T cells (see Ref).
In B Cell ImmunoSpot® Assays, the membrane is coated with the antigen of interest. As the rare antigen-specific B cells present in PBMC secrete antibody, it becomes captured by the membrane-bound antigen around each specific antibody-secreting B cell (ASC) revealing their frequency in the cell material tested. Immunoglobulin class/subclass-specific detection antibodies permit to define the type of antibody that has been produced by the ASC (see Ref). The morphology of the individual spot provides insights into the affinity of the antibodies produced by the individual ASC (see Ref).
ImmunoSpot® Assay’s Unique Sensitivity for Detecting Rare Cells.
ImmunoSpot® Assays can reliably detect antigen-specific T or B cells in frequencies as low as 0.0001% (see Ref), in a frequency range that is barely accessible to flow cytometry-based tests, including intracytoplasmic cytokine staining (ICS) or multimer analysis (tetramers, pentamers, dextramers).
ImmunoSpot® assays are orders of magnitude more sensitive than any type of supernatant-based testing (e.g. by ELISA or Cytokine Bead Arrays, CBAs, or cytokine arrays). This is due to the immediate capture of the analyte around each secreting cell, before it becomes diluted in the culture supernatant, is absorbed by receptors of bystander cells, or undergoes proteolytic degradation. Moreover, unlike supernatant measurements, ImmunoSpot® assays provide high content information revealing (a) the number of cells that produce the analyte, (b) how much analyte each cell produces, and (c) what analytes are co-expressed by the individual cells (see Ref).
ImmunoSpot®: a Fine-Tuned Immune Monitoring System Offered by CTL
In isolation, neither the best of readers, nor the best of kits, nor antigens, test media, and well-preserved PBMC can warrant successful T or B cell immune monitoring. Success requires that all of the above components are meticulously fine-tuned to each other, and are implemented relying upon carefully developed protocols. CTL is the only entity that offers all these components, fine-tuned and pre-tested, for optimal ELISPOT/FluoroSpot performance. We pride ourselves in sharing our 25+ years of leading expertise in this field with you, helping you succeed with your academic and/or regulated goals.
